
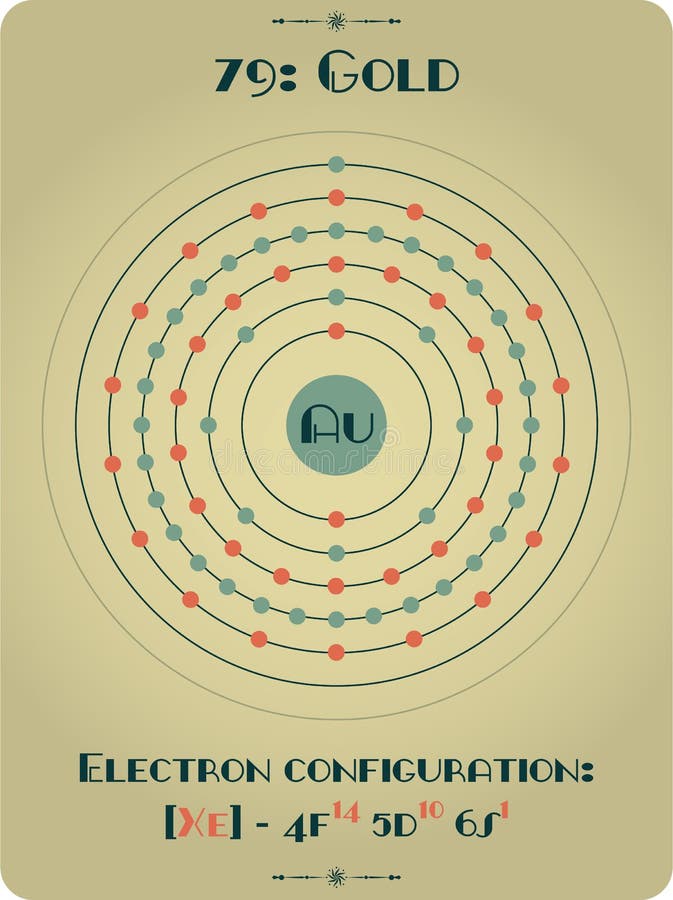
A mixture, on the other hand, is a combination of two or more different substances that are physically intermingled. Gold is not considered a mixture because it consists of only one type of substance: gold atoms. These factors collectively establish gold as an element, distinguishing it from compounds, mixtures, or other forms of matter. The presence of pure gold in nature is evidence of its elemental nature. While it can form compounds with other elements, such as gold chloride or gold oxide, these compounds are not the natural state of gold. Pure and Uncombined: Gold is found in nature primarily as pure, uncombined metal.Gold is represented by the symbol “Au” on the periodic table, derived from its Latin name “Aurum.” 10 11 9 The periodic table is a tabular arrangement of all known elements based on their atomic number and chemical properties. Periodic Table: Gold is listed as an element on the periodic table.8 These properties are a result of its atomic structure, including the arrangement of electrons in energy levels and its electron configuration. Chemical Properties: Gold exhibits unique chemical properties that are characteristic of elements.Gold has an atomic number of 79, indicating that it has 79 protons. Each element has a unique number of protons, which is referred to as the atomic number. Atomic Structure: Gold is composed of atoms that have a specific number of protons in their nucleus.Here are a few reasons why gold is classified as an element:
/139820160-56a131725f9b58b7d0bced18.jpg)
Gold is considered an element because it possesses the fundamental characteristics of an element. This stability is one of the reasons why gold has been treasured and valued throughout history. It exists as pure, uncombined gold in its natural state, and it is chemically stable, meaning it does not readily react with other elements or compounds under normal conditions. While gold can form compounds with other elements, such as gold chloride (AuCl 3) or gold oxide (Au 2O 3), elemental gold itself is not a compound. For example, water (H 2O) is a compound made up of two hydrogen atoms and one oxygen atom. 5 Compounds have specific chemical formulas that describe the types and number of atoms present in the compound. 4Ĭompounds, on the other hand, are substances that are formed when two or more different elements chemically combine together in a fixed ratio. 3 Gold is represented on the periodic table with the symbol “Au” and has an atomic number of 79, which means it has 79 protons in its nucleus. An element is a substance that cannot be broken down into simpler substances by chemical means. Gold is not considered a compound because it is an element.


 0 kommentar(er)
0 kommentar(er)
